OSHIMORI LAB
Laboratory of Cancer Stem Cells and the Niche Microenvironment
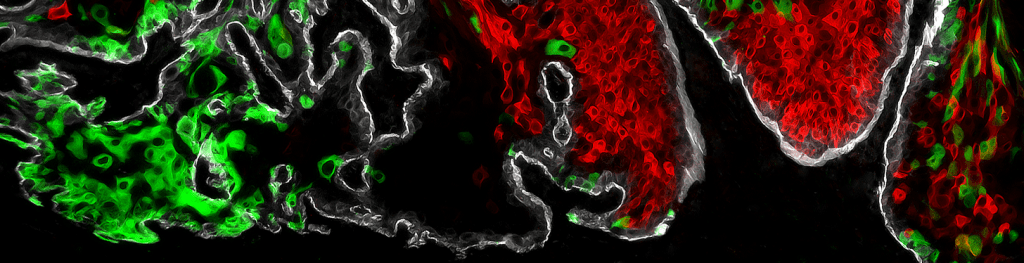
Cancer sometimes comes back after treatment, which can be life-threatening. To make cancer treatment more effective, we focus on cancer stem cells (CSCs) and the niche microenvironment to explore the mechanism of tumor progression and treatment resistance that leads to innovative therapeutic strategies.
Why Cancer Stem Cells?
Many cancer drugs kill rapidly dividing tumor cells, which often reduce tumor burden down to undetectable levels. However, months or years after treatment, a significant number of patients suffer from relapse of more aggressive tumors. The risk of cancer recurrence frightens patients for years. Our incomplete understanding of therapeutic resistance will continue to jeopardize patients’ lives. Cancer stem cells (CSCs) are a small subset of tumor cells sharing similar characteristics as normal stem cells. CSCs have unique abilities to evade cancer treatment and the host immune system. Even though small numbers of CSCs may remain after treatment, they can lead to the revival of tumors by their remarkable properties of self-renewal and multi-lineage differentiation. Therefore, CSCs are considered the Achilles’ heel to improve treatment efficacy and ultimately eradicate cancer. However, methods for destabilizing CSCs have not been as evident as was initially hoped. There is a significant knowledge gap about how CSCs can be so resistant to drug treatment and refractory to the host immune system.
Our Strategy for Destabilizing Cancer Stem Cells
Normal stem cells are maintained by physical and biochemical cues derived from specialized microenvironments or stem cell niches. It has become clear that CSCs are harbored in the unique tumor microenvironment, the so-called CSC niche, comprised of surrounding tumor cells, stromal cells, and immune cells. And, soluble factors and the extracellular matrix emanating from the niche cells regulate the self-renewing proliferation, survival, differentiation, and migration of CSCs. Therefore, our lab focuses on the crosstalk between CSCs and their niche and identifies the critical mechanism that maintains the stem-like state of CSCs and the malignant phenotypes of their progeny. A better mechanistic understanding of the CSC–niche crosstalk could accelerate the development of durable cancer therapeutics.
Squamous Cell Carcinoma – Deadly Cancers Arising from Keratinocytes
Squamous cell carcinomas (SCCs) represent the most frequent human solid tumors. SCCs arise from stratified epithelial cells found on the skin, nasal cavity, oropharynx, thyroid, esophagus, lung, prostate, bladder, and anogenital regions. Skin SCCs are often detected early and treatable. However, a subset (5%-20%) of patients exhibits a more aggressive clinical trajectory, including higher recurrence and metastatic rates. Similarly, high-risk head and neck SCCs (HNSCCs) have high rates of local recurrence, distance metastasis, and death. It is currently difficult to identify the high-risk subsets of SCCs due to the extensive genetic and molecular heterogeneity of tumor cells. Therefore, an improved mechanistic understanding of early-stage SCC is urgently needed to identify targets for prognostic assessment of at-risk populations and early therapeutic intervention.
